RADIOACTIVITY
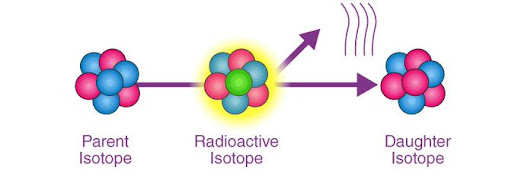
Radioactive decay is the process by which an unstable atomic nucleus loses energy by emitting radiation, which can be in the form of alpha particles, beta particles, gamma rays, or other particles. There are three main types of decays: alpha decay, beta decay, and gamma decay.
Alpha decay In this, an atomic nucleus emits an alpha particle, which is made up of two protons and two neutrons. This reduces the atomic number of the nucleus by 2 and the mass number by 4. Alpha decay typically occurs in heavy elements like uranium, plutonium, and radium.
Beta decay In this a neutron in the nucleus is transformed into a proton and an electron(beta particle). This increases the atomic number of the nucleus by 1 but does not change the mass number.Beta decay occurs in isotopes that have too many neutrons or not enough to be stable
There are two types of beta decay: 1. Beta minus decay (also known as electron emission): In beta minus decay, a neutron is transformed into a proton and an electron, which is emitted from the nucleus. The atomic number increases by 1, and the mass number remains the same.
2. Beta plus decay (also known as positron emission): In beta plus decay, a proton is transformed into a neutron and a positron, which is emitted from the nucleus. The atomic number decreases by 1, and the mass number remains the same.
Gamma decay
In this a nucleus in an excited state releases energy in the form of a gamma ray(high-energy photon). Gamma decay does not change the atomic number or the mass number, but it can occur after alpha or beta decay, as the nucleus may still be in an excited state.
In addition to these three main types of decay, there are other types of decay, such as neutron decay, electron capture, and spontaneous fission. However, these types of decay are much less common than alpha, beta, and gamma decay.
Electron Capture
Neutron Decay
Spontaneous Fission








Comments
Post a Comment