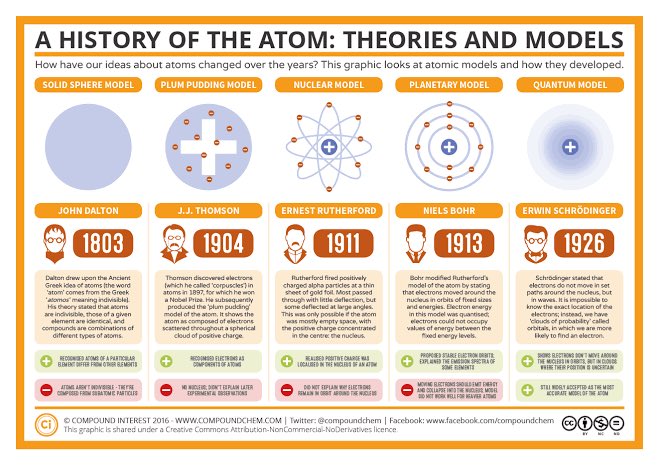
ATOMIC MODELS
Throughout history, scientists have proposed various models to depict the structure of the atom, aiming to unravel its mysteries. Join me as we journey through some of the notable atomic models that have shaped our understanding of the microscopic world.
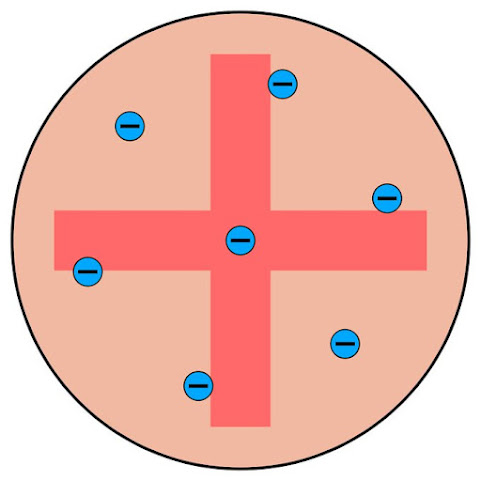
1. Thomson's Plum Pudding Model Proposed by J.J. Thomson in 1904, this model depicted the atom as a positively charged sphere with negatively charged electrons embedded within it. It suggested a more uniform distribution of charge throughout the atom.
2. Rutherford's Nuclear Model Introduced by Ernest Rutherford (1911),this model revolutionized our understanding of atomic structure. It proposed that the atom consists of a tiny, dense,positively charged nucleus orbited by negatively charged electrons.
3. Bohr's Planetary Model In 1913, Niels Bohr expanded upon Rutherford's model. He proposed that electrons occupy specific energy levels or shells around the nucleus, and they can transition between these levels by absorbing or emitting energy.
4. Quantum Mechanical Model Developed in the 1920s, this model, also known as the Wave Mechanical Model, describes the behavior of electrons using mathematical equations called wave functions. It provides a statistical probability distribution of an electron's position.
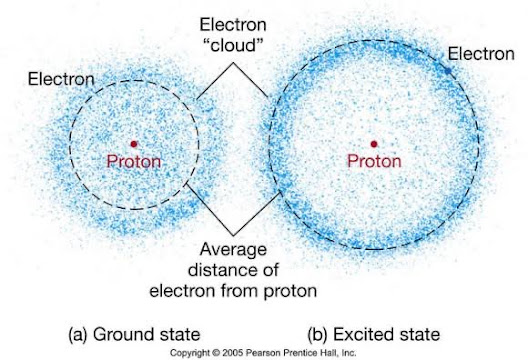
5. Electron Cloud Model An extension of the Quantum Mechanical Model, it represents the probability distribution of finding electrons within certain regions called atomic orbitals. It visualizes electrons as a "cloud" of probability rather than specific paths.
6. Wave-Particle Duality Proposed by Louis de Broglie and supported by experiments, this concept suggests that particles like electrons exhibit both wave-like and particle-like behavior. It challenged traditional notions of particles as discrete entities.
7. Standard Model The Standard Model of particle physics describes fundamental particles and their interactions. It incorporates the understanding of quarks, leptons, gauge bosons, and the Higgs boson, providing a comprehensive view of particles.
8. String Theory Although not an atomic model per se, String Theory is a theoretical framework that suggests particles are not point-like but instead tiny, vibrating strings. It attempts to unify quantum mechanics and general relativity.
These are just a few examples of the diverse atomic models proposed throughout history. Each model has contributed to our understanding of the atom, and newer models have built upon previous ones. The journey to comprehend the intricacies of the atom continues.







Comments
Post a Comment